Mifepristone complications are a growing concern among healthcare professionals and women considering abortion options. Often known as the abortion pill, mifepristone is used in conjunction with misoprostol to terminate pregnancies and manage early miscarriages. Recent study findings reveal a staggering rate of serious adverse events, with some estimates indicating that the incidence is 22 times higher than what is reported by the FDA. Reports have shown that nearly 11% of women experienced complications such as infection, hemorrhage, or other life-threatening issues after taking this medication. As discussions around abortion pill side effects intensify, it becomes crucial to consider the implications of chemical abortion risks and the safety measures that have been subject to changes over recent years.
The complications associated with the use of mifepristone, commonly referred to as the abortion medication, are increasingly coming to light. This chemical abortion method, often combined with another drug, misoprostol, has raised alarms regarding serious side effects that many women may not be fully aware of. As more research sheds light on the potential risks, including significant adverse events that surpass official FDA safety standards, the need for informed consent becomes imperative. Recent findings suggest that the safety of this medication is being called into question, highlighting the urgency for transparency in medical guidelines. Understanding the nuances of medication-induced abortion and its complications could significantly affect women’s health decisions across the country.
Understanding Mifepristone Complications
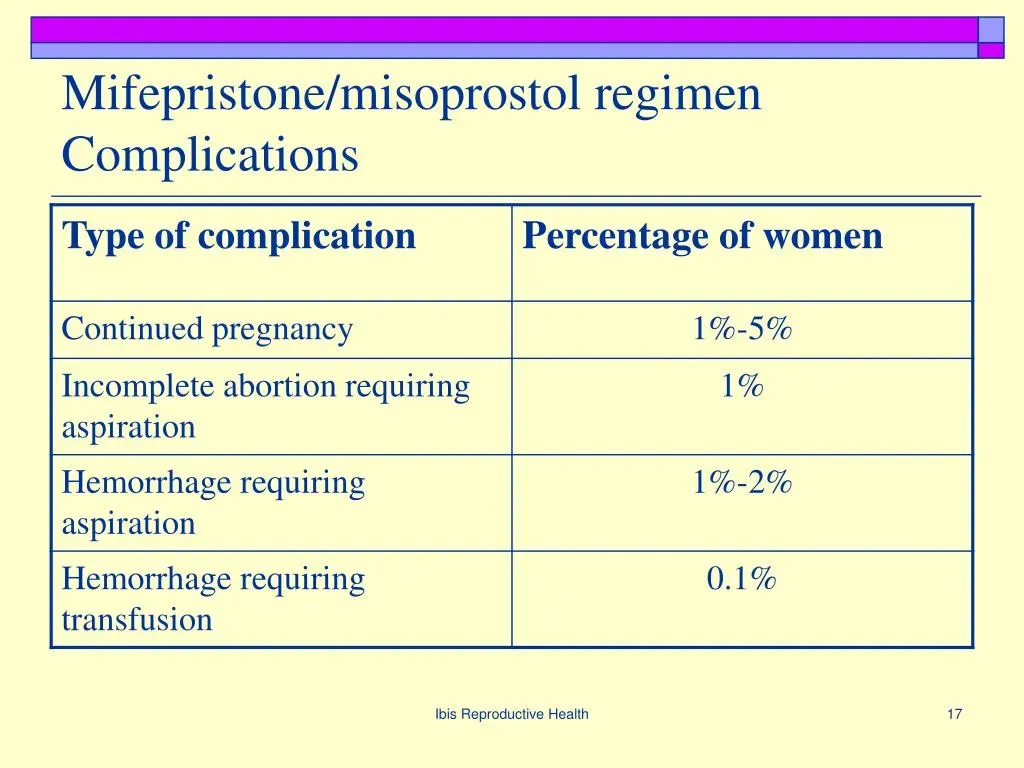
Mifepristone, the active component of the abortion pill regime, has recently been under scrutiny due to reported complications that far exceed initial expectations. A study highlighted that the complications associated with mifepristone use, such as infections and hemorrhages, occur significantly more frequently than the FDA’s approved labels suggest. With estimates showing nearly 11% of women may experience severe adverse events, this revelation challenges the narrative previously held about its safety profile during medical abortions.
The potential for serious mifepristone complications raises important questions about patient safety and informed consent. Unlike the clinical trials that initially informed the drug’s approval, real-world data from hundreds of thousands of cases indicates that risks are much higher than anticipated. These alarming statistics illustrate a need for further investigation into the safety protocols around mifepristone, especially given that reports indicate women are suffering seriously from effects that were not prominently highlighted in early safety assessments.
The Rise of Medication Abortion and Associated Risks
With the increasing accessibility and acceptance of medication abortions in the U.S., estimated to account for 63% of all abortions in 2023, it is crucial to address the associated risks. The use of mifepristone and misoprostol has surged in popularity, reflecting a shift in reproductive health choices. However, as usage spreads, so too do the reports of severe complications, which include serious adverse events that challenge the notion that these medications are a safe alternative to surgical abortion.
The findings from the recent study, which report a complication rate alarmingly higher than those established in controlled clinical environments, underscore the urgent need for clinicians to provide comprehensive information about potential risks. This call to action aligns with the responsibilities of healthcare providers to ensure informed consent, allowing women to make educated choices regarding their reproductive health care, especially regarding the chemical abortion risks associated with mifepristone.
As practitioners debate the safety of medication abortion, it’s vital to scrutinize the adequacy of current FDA safety issues. Despite mifepristone being widely prescribed, many physicians and medical professionals are increasingly advocating for a re-evaluation of existing safety guidelines. The rise in serious adverse events emphasizes the importance of reassessing medication protocols to prioritize the health and safety of women undergoing medication abortions.
FDA Safety Issues and Public Health Concerns
The recent study’s findings pose significant challenges to the FDA’s previous assessments of mifepristone’s safety. Researchers noted a staggering discrepancy between clinical trial results, which showed less than 0.5% severe adverse reactions, and real-world outcomes that suggest a rate of 11%. This divergence raises serious moral questions about the agency’s ongoing approval of mifepristone and the adequacy of its surveillance mechanisms to ensure patient safety in a rapidly evolving landscape of reproductive health.
Public health concerns regarding the use of mifepristone are palpable. As advocates call upon the FDA to reinstate stricter safety measures that were relaxed under recent administrations, it is essential to highlight the potential life-threatening complications reported in ongoing studies. Addressing these issues can lead to improved regulatory frameworks meant to safeguard women’s health, thereby ensuring that those choosing medication abortions do so with a full understanding of the risks they may encounter.
Mifepristone Study Findings: A Wake Up Call
The recent mifepristone study conducted by the Ethics & Public Policy Center has emerged as a critical wake-up call for both medical professionals and regulatory bodies. By analyzing comprehensive insurance claims data from over 865,000 medication abortions, the study reveals a startling reality about the safety of the abortion pill. The findings indicate that serious complications from mifepristone use are shockingly common, contradicting the reassuring narrative often provided to patients and healthcare providers.
These study findings also highlight the urgent need for a re-evaluation of existing medical guidelines surrounding abortion pills. Women deserve to be informed not just about the efficacy of the abortion procedure but also about potential complications that are significantly more prevalent than previously reported. This research underscores the need for transparency and comprehensive education on the risks associated with mifepristone to better prepare women for potential adverse outcomes.
Informed Consent and Women’s Health
Informed consent forms a cornerstone of ethical medical practice, particularly in high-stakes areas like reproductive health. Given the new study’s findings regarding mifepristone, it becomes imperative that clinicians ensure women are made fully aware of the potential risks associated with using the abortion pill. The reality that nearly 11% of women experience severe complications invokes a moral imperative for healthcare providers to communicate these risks upfront, allowing women to make truly informed decisions about their reproductive health.
The overwhelming complexity of these decisions necessitates a strong commitment to patient education. As new data continues to emerge around mifepristone and its associated risks, there must be a collective effort to improve informed consent processes. This emphasis on communication could significantly influence women’s experiences, ensuring they enter into these medical decisions with a comprehensive understanding of all potential risks involved in their choices.
The Role of Medical Professionals in Managing Abortion Pill Risks
Healthcare providers play a vital role in managing the risks associated with mifepristone and ensuring patient safety during medication abortions. The responsibilities of medical professionals extend beyond simply prescribing the abortion pill, encompassing an obligation to educate patients about possible side effects and complications. This includes understanding the range of adverse reactions documented in studies and ensuring that patients are prepared for their potential experiences.
As studies reveal alarming data about serious adverse events, it is increasingly crucial for healthcare providers to stay informed and communicate openly with their patients. A proactive approach in discussing the risks of mifepristone can mitigate negative outcomes and empower women to make informed decisions about their reproductive health. Continuous training and dissemination of recent findings will be key in equipping medical professionals to handle these conversations judiciously.
Comparative Analysis of Clinical Trials and Real-World Data
The discrepancy between historical clinical trial results and contemporary real-world data regarding the use of mifepristone calls for a thorough comparative analysis. Initial trials suggested that serious adverse events were exceedingly rare, indicating a safety profile that may no longer be accurate based on the emerging evidence. This divergence points to a need for updated studies that reflect the complexities of current medical practices and societal changes surrounding abortion.
By examining the differences between clinical trials and actual patient experiences, clinicians and researchers can develop a more nuanced understanding of mifepristone’s safety. This understanding is essential not only for future regulatory measures but also for the formulation of effective guidelines that align with the realities faced by women undergoing medical abortions today. Continuous scrutiny will ensure that mifepristone usage is both safe and effective in practice.
Advocacy for Restored Safety Precautions
The findings from the mifepristone study have prompted strong advocacy for reinstating original safety precautions that were in place prior to recent regulatory rollbacks. Experts and researchers are calling for these safety measures to be revived to mitigate the risks associated with the abortion pill. The evidence indicates that, without stringent protocols, patients face significant dangers that could be better managed through clear and enforceable regulations governing the use of mifepristone.
This call to action emphasizes the need for protecting women’s health as a priority in the evolving landscape of reproductive rights. Restoring FDA safety guidelines could provide a safety net for women, ensuring that they have access to safe abortion practices while being fully informed of associated risks. Advocacy for such changes is paramount to uphold the integrity of reproductive health services and ensure the well-being of individuals who may rely on medical abortions.
The Future of Mifepristone and Women’s Health Policy
As the conversation around mifepristone continues to evolve, it is essential to consider the implications for women’s health policy moving forward. The ongoing discussions about the abortion pill’s safety and efficacy may influence future regulations and healthcare practices concerning reproductive health. Policymakers must take these findings seriously and work toward establishing frameworks that prioritize patient safety and informed consent, reflecting the realities highlighted by recent studies.
The future trajectory of mifepristone usage, along with broader reproductive health policies, may hinge on how stakeholders respond to new evidence of complications. Creating a comprehensive approach that integrates patient safety, access to information, and careful monitoring of medication use may ensure that women can exercise their reproductive rights while minimizing harmful outcomes. As such, the collaboration between healthcare providers, regulatory bodies, and advocacy groups is vital in shaping the future landscape of women’s health.
Frequently Asked Questions
What are the common mifepristone complications reported by users?
Common mifepristone complications include serious adverse events such as infections, hemorrhages, and other life-threatening conditions reported by nearly 11% of women who underwent a chemical abortion, according to a recent study.
How do mifepristone complications compare to FDA-approved rates?
A recent study highlights that the rate of serious complications associated with mifepristone is 22 times greater than what is documented on the FDA-approved drug label, which reflects data from clinical trials conducted decades ago.
What serious adverse events are associated with mifepristone use?
Serious adverse events related to mifepristone use include significant health issues such as severe infections and uncontrollable bleeding, affecting over 11% of patients based on analysis of insurance claims data.
What are the FDA safety issues surrounding mifepristone?
FDA safety issues concerning mifepristone have been raised due to the relaxation of earlier safety measures, leading to increased reports of serious complications, which the recent study suggests are considerably higher than previously reported.
What do mifepristone study findings indicate about its risks?
Mifepristone study findings reveal alarming risks, showing that serious complications occur at significantly higher rates than those published by the FDA, indicating a potential public health crisis that necessitates urgent investigation.
What should women know about the abortion pill side effects and risks?
Women should be informed that mifepristone, while legally sanctioned for abortion, carries risks of serious side effects such as infections and hemorrhaging, and the negative outcomes are reportedly occurring at higher rates than previously established.
Why is the latest research on mifepristone important for public health?
The latest research on mifepristone is crucial for public health as it reveals a substantial discrepancy between reported safety and real-world complications, supporting calls for a reevaluation of current FDA guidelines and informed consent practices.
How many women are estimated to have experienced serious complications from mifepristone?
Based on recent estimates by health professionals, approximately 71,000 women may have experienced serious complications from mifepristone in 2023, underscoring the need for increased awareness and safety measures.
What are the implications of the study’s findings for future mifepristone regulations?
The implications of the study’s findings for future mifepristone regulations include calls for reinstating previous FDA safety measures and ensuring that women receive comprehensive information regarding the potential risks associated with the abortion pill.
What challenges exist in understanding mifepristone complications?
Challenges in understanding mifepristone complications stem from the reliance on insurance claims data, which may not accurately reflect causal relationships between the drug and adverse medical outcomes.
| Key Points |
|---|
| A new study reveals serious adverse events associated with mifepristone, the abortion pill. |
| Mifepristone is used to terminate pregnancies and manage early miscarriages. |
| The study shows serious side effects occur at a rate 22 times higher than indicated by the FDA label. |
| Nearly 11% of women experienced serious complications after medication abortions. |
| The study analyzed over 865,000 medication abortions from 2017 to 2023. |
| Previous FDA trials indicated severe reactions in less than 0.5% of participants. |
| Calls are being made for the FDA to reinstate original safety measures for mifepristone. |
| There is concern about the increasing complications following the lifting of essential safety guidelines. |
| Experts argue for informed consent regarding the risks associated with mifepristone. |
| Mifepristone is reported as one of the safest medications, yet scrutiny continues. |
Summary
Mifepristone complications have emerged as a serious concern according to a recent study highlighting a significant increase in adverse events associated with the abortion pill. The findings suggest that nearly 11% of women experience complications such as infections and hemorrhage, revealing a discrepancy between reported and actual risks. This alarming data calls for renewed attention to safety regulations surrounding mifepristone, emphasizing the need for informed consent for women considering this medication. As ongoing discussions unfold, it is crucial to prioritize women’s health and reevaluate the protocols governing its use.