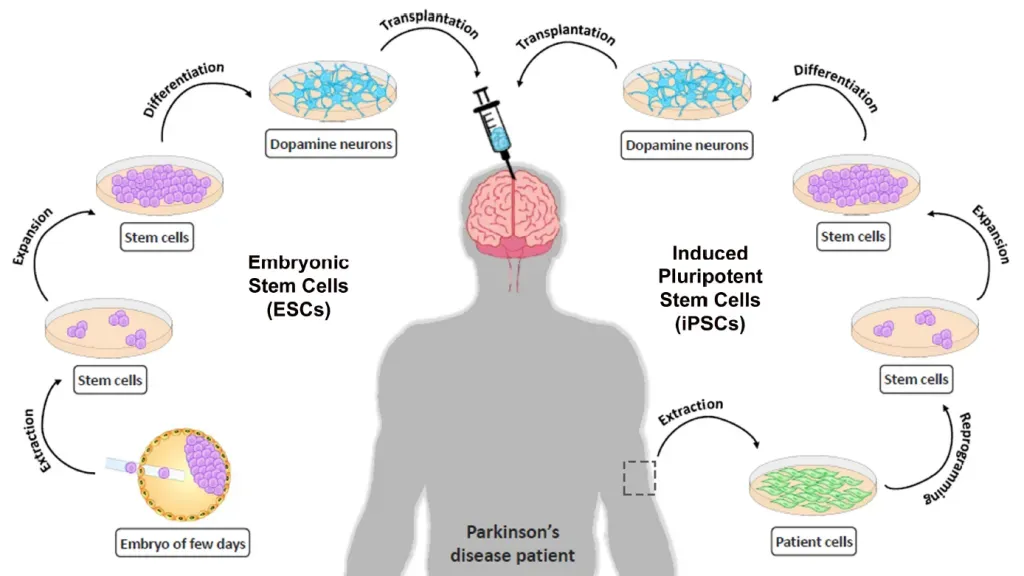
Stem cell therapy for Parkinson’s is emerging as a groundbreaking approach in the quest for effective Parkinson’s disease treatment, showcasing strong promise in alleviating debilitating symptoms. With an estimated one million Americans affected by Parkinson’s, the need for innovative solutions is more urgent than ever. Recent studies at Memorial Sloan Kettering Cancer Center have demonstrated how stem cells can be used to regenerate nerve cells that are crucial for dopamine production—a neurotransmitter that significantly influences movement and coordination. In a clinical trial for Parkinson’s involving 12 patients, researchers reported notable improvements in symptoms, indicating that this therapy could revolutionize how neurological disorders are managed. As the research progresses towards larger-scale trials, it’s clear that this advancement in stem cell research has the potential to transform lives and provide hope for many.
The exploration of stem cell treatment for Parkinson’s is a significant development in modern medicine, particularly for those grappling with this challenging neurological condition. This innovative therapy aims to restore lost motor functions by using specialized cells to replace damaged neurons responsible for dopamine production. As many patients seek alternatives to traditional therapies, which often involve increasing doses over time, this approach could offer a more sustainable solution. The promising results from recent clinical trials highlight that these cellular therapies not only focus on managing symptoms but also hold the potential for reversing damaging effects in the brain. Such advancements pave the way for greater breakthroughs in Parkinson’s management and open new avenues in the fight against other neurological disorders.
Understanding Parkinson’s Disease and Its Treatment Options
Parkinson’s disease is a progressive neurological disorder that primarily affects movement. The hallmark symptoms include tremors, stiffness, balance issues, and difficulty with coordination, largely attributed to reduced dopamine production in the brain. Current traditional treatment options, such as levodopa, aim to alleviate these symptoms but often become less effective as the disease progresses. As the condition deteriorates, patients frequently find themselves in need of increased dosages and face significant side effects, such as dyskinesias. Consequently, researchers are actively exploring innovative treatment options that may provide better outcomes for those impacted by this debilitating disorder.
Among these potential treatments, stem cell therapy stands out as a beacon of hope. By utilizing stem cells to generate new dopamine-producing neurons, there is a chance to improve motor function and slow the progression of the disease. The ability to replace lost dopaminergic neurons represents a significant advancement in the field of Parkinson’s disease treatment, with research continually accelerating. With approximately one million individuals diagnosed with Parkinson’s in the U.S. and thousands of new cases emerging each year, the urgency to develop safe and effective treatments has never been more critical.
Recent Advances in Stem Cell Therapy for Parkinson’s
Recent breakthroughs in stem cell therapy have shown strong promise, particularly from the phase 1 clinical trial conducted by researchers at Memorial Sloan Kettering Cancer Center. In this study, the introduction of embryonic stem cells into the brains of Parkinson’s patients led to significant improvements in their symptoms over an 18-month period. By regenerating neurons that produce dopamine, these stem cells not only addressed the deficiency responsible for many Parkinson’s symptoms but did so with a favorable safety profile, which is critical in considering future applications of this treatment.
The findings spotlight how stem cell therapy could revolutionize Parkinson’s disease treatment. Patients in the higher dosage group reported an impressive 2.7 hours of additional ‘on time’ each day, indicating significant improvements in their ability to function normally with fewer symptoms. This form of therapy paves the way for potentially halting disease progression and enhancing motor skills, making it a leading edge in contemporary research on neurological disorders. Ongoing investigations will be essential to validate these promising results and possibly expand treatment options for millions suffering from Parkinson’s.
Future directions include moving to larger phase 3 clinical trials, which are crucial for determining efficacy on a wider patient base. The FDA’s approval for further testing reflects confidence in the therapy’s potential, and the prospect of developing a viable, effective stem cell treatment holds significant hope for the future of Parkinson’s disease management.
The Role of Dopamine in Parkinson’s Disease
Dopamine is a vital neurotransmitter that plays a crucial role in coordinating movement and maintaining balance. In Parkinson’s disease, there’s a marked decline in the production of dopamine due to the degeneration of neurons in the brain. Each time a dopamine-producing cell dies, individuals exhibit increasingly severe symptoms that can dramatically affect their quality of life. Understanding the fundamental role that dopamine plays in this disorder is essential not only for patients but also for developing effective treatments that can restore function and improve daily living activities.
Stem cell therapy targets the core issue by introducing new neurons capable of producing dopamine. This breakthrough approach aims not only to relieve symptoms temporarily but to effect lasting change within the neurological framework of patients. For many, this could mean a return to normalcy and independence, drastically changing their day-to-day experiences. Future research also explores how enhancing dopamine production through various methodologies could blend with traditional treatments for a synergistic effect, further enhancing patient outcomes.
Clinical Trials and Their Importance in Stem Cell Research
The process of testing new therapies involves rigorous clinical trials, and in the realm of stem cell therapy for Parkinson’s disease, these trials are critical for success. The phase 1 trial at Memorial Sloan Kettering has paved the way for a future phase 3 trial, emphasizing the importance of clinical validation in proving the safety and effectiveness of such treatments. These trials not only help identify potential risks and adverse reactions but also refine dosages and treatment regimens to optimize patient care. For any stem cell application to become a mainstream therapy, it must undergo strict scrutiny through these clinical evaluation phases.
Engaging larger groups of participants in subsequent trials allows researchers to gather more comprehensive data, leading to more robust conclusions. Not only does this foster greater trust in the research within the broader medical community, but it also builds confidence among patients who are anxious for promising treatment options. Ensuring that stem cell therapies pass rigorous testing phases reinforces their potential as a new standard for treating neurological disorders long term.
Potential Risks of Stem Cell Therapy
While the potential benefits of stem cell therapy for Parkinson’s disease are enticing, it is important to approach the topic with a balanced view, acknowledging the potential risks involved. The immune suppression that often occurs prior to the implantation of stem cells is one area of concern. This suppression is crucial to prevent rejection of the transplanted cells but can leave patients susceptible to infections and other complications. Therefore, a careful assessment of each patient’s risk profile is necessary before pursuing stem cell therapy.
In addition to immune suppression, the long-term effects of stem cell injections remain largely unknown. Although early trials have indicated safety and initial efficacy, comprehensive observations over extended periods will be essential to ensure that patients do not experience unforeseen complications or regression in their condition. This inherent uncertainty necessitates thorough pre- and post-treatment assessments, as well as ongoing monitoring of patients who undergo these innovative therapies.
Future Directions in Stem Cell Research for Neurological Disorders
The exciting advancements in stem cell research have implications that reach far beyond just Parkinson’s disease. As scientists expand their understanding of neurological disorders and how stem cells can be harnessed, there is potential for breakthroughs in the treatment of a variety of conditions, including Alzheimer’s disease, multiple sclerosis, and spinal cord injuries. Research teams are increasingly focused on the mechanisms of neuroregeneration, aiming to develop therapies that not only replace lost cells but also promote overall brain health and functionality.
The integration of stem cell therapy into mainstream medicine represents a paradigm shift, particularly in how we view disease management in neurology. As trials continue and methodologies evolve, we can expect a more profound understanding of how to genetically tailor therapies for individual patients. A future where stem cell therapy is a predominant treatment for a variety of neurological disorders seems within reach, advocating for continuous investment in research and development.
Understanding Levodopa’s Limitations in Parkinson’s Therapy
Levodopa has long been considered the gold standard for treating Parkinson’s disease. It is converted into dopamine in the brain, effectively managing symptoms for many patients. However, as the disease progresses, the effectiveness of levodopa diminishes, leading to an escalation in required dosages. This cycle poses challenges for patients who may encounter periods of off time where symptoms re-emerge, such as stiffness or the aforementioned dyskinesias. Consequently, there is a pressing need for alternative or complementary therapies that can address the shortcomings of levodopa.
Given the complex interplay of Parkinson’s disease progression and levodopa treatment efficacy, researchers are now exploring synergistic approaches that combine levodopa with innovative therapies like stem cell treatment. These combinations could help manage symptoms more effectively while delaying the decline of motor function. The goal is to create a comprehensive treatment landscape that not only maximizes the current therapeutic strategies but also integrates cutting-edge therapies, thereby improving the overall quality of life for patients.
Patient Perspectives on Emerging Therapies
Patients diagnosed with Parkinson’s disease often find themselves navigating a complex treatment landscape muddled with uncertainty and trial-and-error approaches. As new therapies emerge, such as stem cell treatment, the prospect of enhanced quality of life fosters hope among patients and caregivers alike. Understanding how these therapies may effectively address symptoms and alter the trajectory of the disease encourages broader patient involvement in clinical trials, promoting a community eager to participate in advancing their health.
Moreover, it is essential to consider patient perspectives on risk versus reward when it comes to exploring novel treatment options. In discussions about potential therapies, including stem cell therapy, patients desire transparent communication from their healthcare providers regarding the scientific evidence, potential outcomes, and any associated risks. This patient-centric approach not only empowers individuals to make informed decisions about their own health but also fosters a supportive environment that can significantly impact attendance and enthusiasm for participation in clinical studies.
The Intersection of Science and Ethics in Stem Cell Research
The ethical considerations surrounding stem cell research remain a topic of heated debate within both the scientific community and the general public. The use of embryonic stem cells, for instance, raises questions regarding the moral implications of using a human embryo for research purposes. Advocates stress the potential lifesaving effects of such research in treating debilitating diseases, while opponents highlight the ethical concerns in the destruction of embryos. As scientific exploration unfolds, establishing a framework that respects both the needs of research and ethical considerations is paramount.
Additionally, regulatory bodies must navigate the complex landscape of stem cell research to ensure that advancements are made responsibly. This includes fostering guidelines that promote safe and effective treatments while safeguarding patient rights and moral standards. Reaching a consensus on these issues is imperative, as stakeholders—including researchers, policymakers, and the public—collaborate to balance innovation with ethical responsibility in the ever-evolving field of stem cell therapy for neurological disorders.
Frequently Asked Questions
What are the benefits of stem cell therapy for Parkinson’s disease?
Stem cell therapy for Parkinson’s disease has shown potential benefits in improving motor functions and reducing symptoms. In a recent clinical trial, patients reported significant improvements in ‘on time,’ indicating reduced symptoms and enhanced daily functioning. The treatment involves the injection of stem cells that produce dopamine, which is crucial for movement and coordination, addressing one of the main challenges of Parkinson’s.
How does stem cell therapy for Parkinson’s work?
Stem cell therapy for Parkinson’s involves the use of stem cells derived from embryos, which are developed into neurons that produce dopamine. These cells are injected into the brain to replace lost dopaminergic neurons, helping alleviate symptoms associated with Parkinson’s disease. This approach targets the core issue of dopamine deficiency, potentially leading to improved motor functions.
Are there any risks associated with stem cell therapy for Parkinson’s?
Yes, stem cell therapy for Parkinson’s disease does involve certain risks, including the need for immune suppression prior to treatment and potential complications from the procedure itself. While early studies have reported safety in cell implantation, further extensive trials are necessary to evaluate both safety and long-term efficacy in a larger patient population.
What results have been reported in clinical trials for stem cell therapy for Parkinson’s?
In a phase 1 clinical trial, patients receiving stem cell therapy for Parkinson’s showed notable improvement in their symptoms, with many reporting increased ‘on time’ and a decrease in their UPDRS scores. After 18 months, there were no serious side effects observed, suggesting that stem cell injections could provide significant benefits in managing Parkinson’s disease.
What is the significance of the FDA’s approval for stem cell therapy clinical trials for Parkinson’s?
The FDA’s approval for a phase 3 clinical trial of stem cell therapy for Parkinson’s is a critical milestone, allowing researchers to assess the treatment’s efficacy in a larger group of around 100 patients. This step is pivotal in determining whether stem cell therapy can become a viable option for treating Parkinson’s disease on a broader scale, potentially leading to significant clinical advancements.
How long does it take to see results from stem cell therapy for Parkinson’s?
Patients in the recent clinical trial for stem cell therapy for Parkinson’s had reported improvements by the 18-month mark. The time to see results can vary, but initial findings suggest that significant benefits can be observed within a year and a half following treatment, depending on individual response and dosage levels.
What are the future prospects of stem cell therapy for treating neurological disorders like Parkinson’s?
Stem cell therapy for Parkinson’s disease represents a promising avenue for treating not only this condition but potentially other neurological disorders as well. Continued research and successful clinical trials could pave the way for novel therapies that repair brain function and improve quality of life for patients suffering from various neurological challenges.
| Key Points | Details |
|---|---|
| Trial Overview | The study was a Phase 1 trial involving 12 patients with Parkinson’s disease, using stem cells derived from early-stage embryos to create neurons for transplantation. |
| Dosage and Effects | Higher-dose patients reported an average of 2.7 extra hours of ‘on time’ daily and showed a decrease of over 20 points on the MDS-UPDRS scale. |
| FDA Approval | Based on positive trial outcomes, the FDA has approved a Phase 3 clinical trial involving around 100 patients. |
| Safety and Efficacy | The injected cells produced dopamine and showed no serious side effects, indicating the therapy’s safety. |
| Expert Opinions | Experts noted the potential for this therapy to not only slow progression of Parkinson’s but to improve motor function significantly. |
| Limitations | The study’s limitations include its small sample size and the need for larger studies to confirm efficacy. |
Summary
Stem Cell Therapy for Parkinson’s is showing strong promise based on recent findings from a clinical study conducted by researchers at Memorial Sloan Kettering Cancer Center. With significant improvements in patient symptoms and movement-related issues reported after 18 months, this breakthrough therapy may revolutionize treatment options for the approximately one million people living with Parkinson’s disease in the U.S. The promising results have encouraged further research, with an upcoming Phase 3 trial to assess the efficacy of this innovative approach, potentially transforming the future outlook for patients suffering from this neurological condition.